NOVAERUS Protect 800 醫療級等離子空氣消毒機
[0.002秒間殺滅空氣中[氣溶膠]的細菌及病毒,包括新冠病毒]
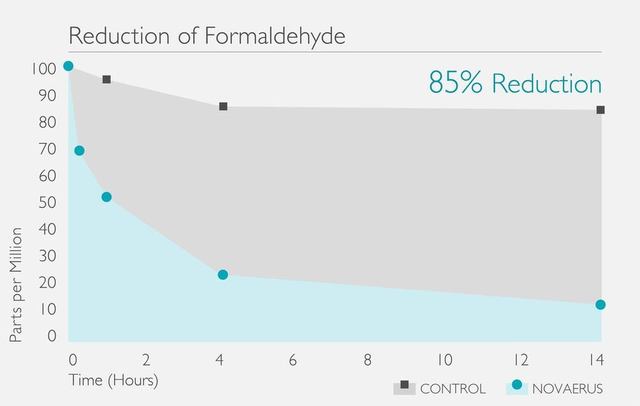
[病毒測試證明Novaerus可減少99.9%空中傳播的病毒]
產品特點
●專為中等室內空間進行連續空氣消毒和氣味控制而設計
●專利超低能量等離子技術
●能夠從DNA的層面上消滅空氣中的病毒和微生物 (包括但不限於冠狀病毒、甲型流感病毒、大腸桿菌、PM2.5等)
●雙速風扇
●可以24/7無間斷運行
●可安裝在牆上或放置在任何表面上
●特別適用於: 病房、手術室、課室、共用空間、護士站、試場等
●生產地: 愛爾蘭 , 2年保養
產品規格
●型號: NOVAERUS NV800
●尺寸: 36.6 (h) x 36.5 (w) x 11.4 (d) 厘米
●重量: 4.7公斤
●風扇風量: 220立方米/小時 和 260立方米/小時
●噪音水平: 40分貝和45分貝
●運作環境: 10-35°C攝氏溫度, 10-75%相對濕度
●質量與安全認證: ISO 9001, ISO 14001 & OHSAS 18001
●建議運作範圍: 120立方米 (約400尺空間) *只需把Novaerus放置放在人群聚集處
Novaerus 技術用於病毒性感染控制的額外資訊
由於病毒非常細小,眾多過濾技術並無法捕獲病毒顆粒,同時亦產生從濾網處二次感染的風
險。Novaerus DBD 等離子體消毒淨化技術是一種非選擇性的高速物理殺滅技術,並不依賴傳
統濾網,提供了一種獨特且安全的24/7 全天侯解決方案來殺滅空氣中散播的病毒,從源頭減
少懸浮和沉澱的病毒顆粒、降低人觸碰病源體的機會,從而大大降低傳染病爆發的風險。
●Novaerus DBD 等離子體消毒淨化技術透過結合一系列物理反應(電穿孔,電子轟擊,蝕刻
等)迅速消滅病原體。美國加州大空總署研究中心NASA Amesr Research Centre 已對此進行了
獨立的測試和證明。
●Novaerus 技術已經針對多種病毒進行了獨立測試,對有囊膜及無囊膜的菌株有一致的殺滅率。
●病毒的基因結構多樣性比植物、動物、古細菌或細菌更繁複,世上存在許多不同類型的病毒。
●我們不可能對所有類型的病毒進行獨立測試。因此,Novaerus 選擇測試一系列對人類有致病
性的病毒。某些病毒(包括有囊膜及無囊膜類)由於太危險而無法進行實驗室測試,Novaerus
亦選擇了類近的病毒作為替代品進行試驗。
●2015 年MERS 疫情傳播到韓國之後,韓國衛生部門決定在救護車輛上廣泛安裝可人機共存的
空氣消毒器Novaerus Protect 200,在救護車輛運送病人的過程中實時對車輛空氣進行消毒,
保護醫護人員安全,並獲得消防處的讚揚信。
**紮根愛爾蘭的Novaerus 公司於2006 年研發專利DBD (Dielectric Barrier Discharge)等離子體消
毒淨化技術應用於醫療級空氣消毒系統,於2008 年在歐洲各地開展一系列臨床試驗,至今已
發表逾30 份臨床測試及實驗室報告、16 份個案報告。Novaerus 系列產品正在協助全球數百家
醫院、護老機構、救護車、學校及各私營機構控制病毒感染的風險。
經過在世界各地進行的多個實驗室測試和臨床試驗,已證明Novaerus等離子技術是安全有效:
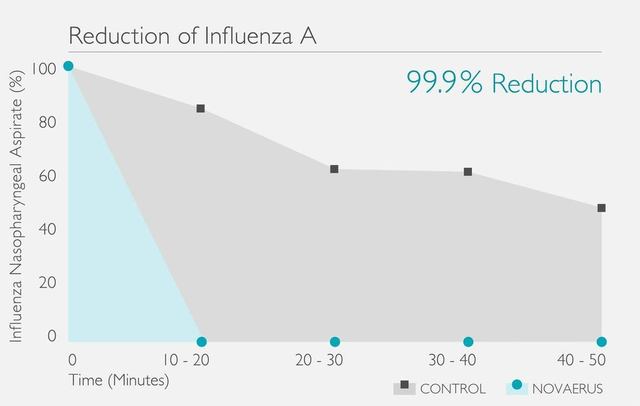
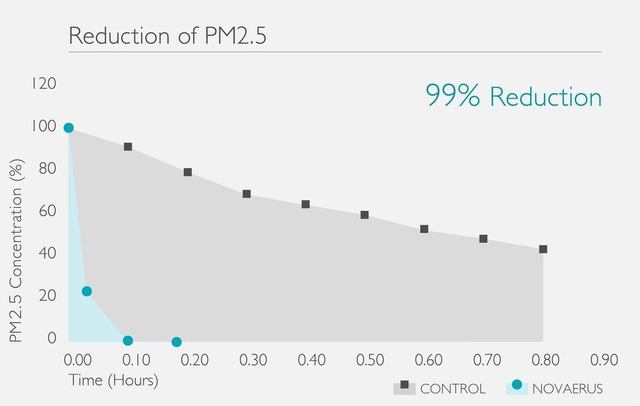
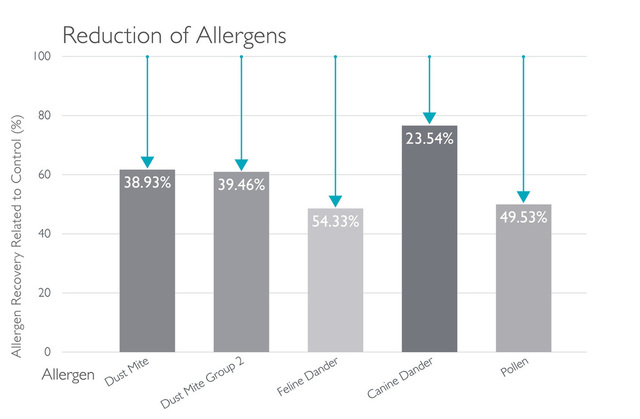
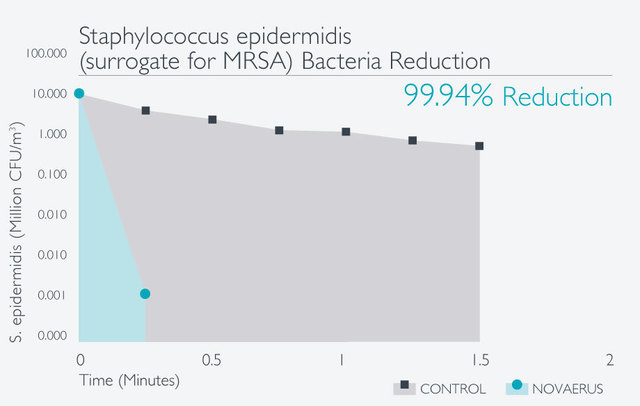
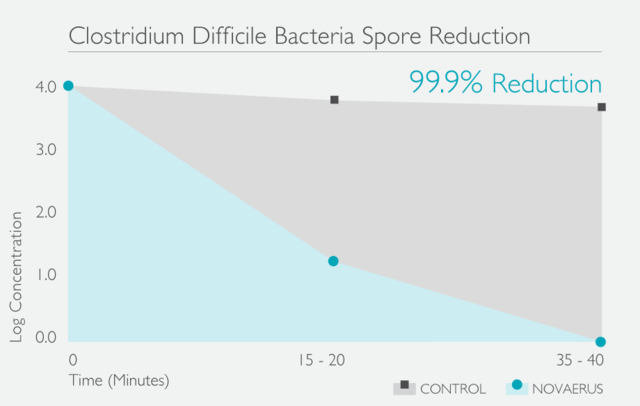
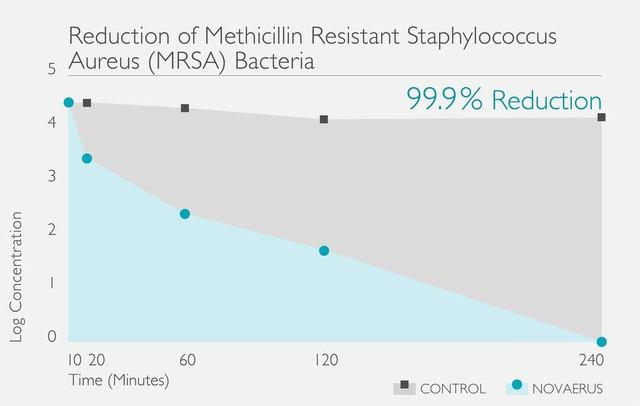
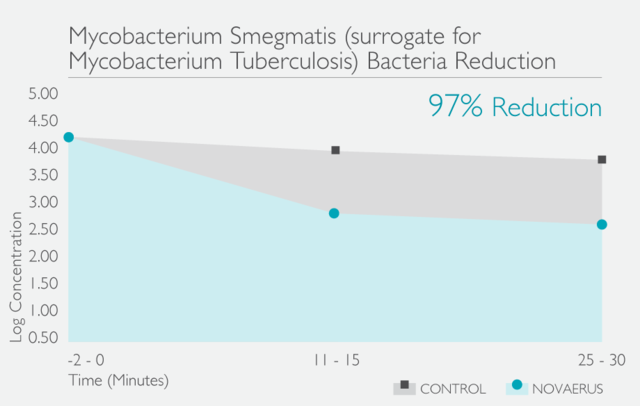
Novaerus與其他空氣過濾機比較:







